Answer: The correct answer is 0.50 mol A left over.
Step-by-step explanation:
Excess reactant is defined as the reactant which is present in excess in the reaction and does not predict the formation of product.
Limiting reactant is defined as the reactant which is present in limiting amount and which limits the formation of products.
For the given chemical reaction:

By Stoichiometry of the reaction:
2 moles of B is reacting with 1 mole of A.
So, 3 moles of B will react with =
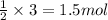 of A.
of A.
As, the given moles of A is more than the required moles. So, it is present in excess and is considered as an excess reagent.
Amount of excess reagent left (A) = 2 - 1.5 = 0.5 moles
Thus, the correct answer is 0.50 mol A left over.