Answer : When magnesium salts combine with the alkaline water it induces precipitation of solid
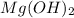 .
.
Explanation :
- Chemical change : The chemical change occurs when a substance combine with the another substance to form a new substance.
It can not be easily reversible.
The new product is formed or precipitate may be formed.
- Physical change : The physical change occurs when a substance combine with the another substance then no new compound is formed.
It can be reversible.
No new product is formed only changes occurs in the state or phase.
As per question, when magnesium salts combine with the alkaline water it induces precipitation of solid
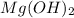 .
.
The balanced chemical reaction is,
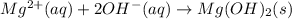
That mean a new compound is formed by this reaction. So, the chemical change occurred in this reaction.