Step-by-step explanation:
It is known that density is mass divided by volume of a substance.
Mathematically, Density =
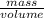
Since, it is given that volume is 69.9 mL and density is 1.78 g/mL.
Hence, calculate the mass as follows.
Density =
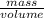
1.78 g/mL =
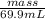
mass = 124.42 g
Thus, we can conclude that mass of potassium chloride is 124.42 g.