Answer:
the ratio of parent to daughter = 15.38 : 1
Step-by-step explanation:
The radioactive disintegration is considered to be first order reaction.
The relation between first order rate constant and the half life is:
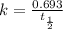
Let us calculate the rate constant from half life as
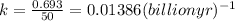
The rate law expression for first order reaction is
![kt=ln([A0])/(At)](https://img.qammunity.org/2018/formulas/chemistry/high-school/8hrgpad673imgqy4ei2tbddq5f44q573bi.png)
Where
A0 = initial amount of rubidium isotope = say 100
At= final amount of rubidium isotope after 4.6 billion years
putting all values
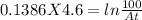
1.065=100/At
At=93.897
The amount of daughter isotope formed =100-93.897 = 6.103
the ratio of parent to daughter = 93.897:6.103 = 15.38 : 1