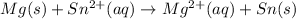Answer:
Step-by-step explanation:
Spectator ions are defined as the ions which does not get involved in a chemical equation or they are ions which are found on both the sides of the chemical reaction present in ionic form.
The given chemical equation is:-
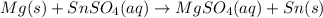
The complete ionic equation is:
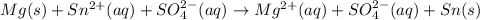
The
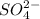 ions which are present on both the sides of the equation are not involved in net ionic equation.
ions which are present on both the sides of the equation are not involved in net ionic equation.
Hence, the net ionic equation is: