Answer: The new pressure of 750 mmHg is greater than initial pressure of 500 mm Hg and the new volume of 6.0 ml is lesser than the initial volume of 9.0 ml.
Step-by-step explanation:
Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
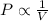 (At constant temperature and number of moles)
(At constant temperature and number of moles)
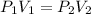
where,
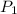 = initial pressure of gas = 500.0 mmHg
= initial pressure of gas = 500.0 mmHg
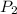 = final pressure of gas= 750.0 mmHg
= final pressure of gas= 750.0 mmHg
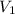 = initial volume of gas = 9.0 ml
= initial volume of gas = 9.0 ml
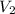 = final volume of gas = ?
= final volume of gas = ?
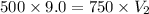
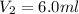
Therefore, the gas would compress to new volume of 6.0 ml.
The new pressure of 750 mmHg is greater than initial pressure of 500 mm Hg and the new volume of 6.0 ml is lesser than the initial volume of 9.0 ml.