Answer:
The sodium hydroxide under equal concentrations
Step-by-step explanation:
Hello,
At first, lets consider the neutralization reactions for the involved acids with sodium hydroxide:
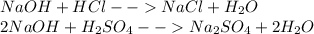
As you can see the mole ration between hydrochloric acid and sodium hydroxide is 1 to 1, it means that for equal concentations, the volume of acid will equal the volume of base. However, for sulfuric acid we have a 2 to 1 ratio (2 moles of hydroxide per 1 mole of acid), it means that for equal concentrations, the half of sulfuric acid's volume will be required to neutralize the sodium hydroxide. In such a way, the hydrochloric acid's solution will require more volume to neutralize the sodium hydroxide under equal concentrations.
Best regards.