Step-by-step explanation:
Temperature is directly proportional to the rate of dissolution.
That is,
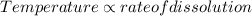
This means that more is the temperature more quickly the solute is able to dissolve in the solvent.
For example, sugar will dissolve in hot water more quickly as compared to water at room temperature.