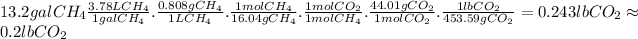Answer:
0.2 lb
Explanation:
The gas used for fuel is mostly methane (CH₄), with a density of about 0.808 g/L. The combustion reaction is:
CH₄ + 2 O₂ → CO₂ + 2 H₂O
We can establish the following relations:
- 1L of CH₄ has a mass of 0.808 g.
- The molar mass of CH₄ is 16.04 g/mol.
- The molar ratio of CH₄ to CO₂ is 1:1
- The molar mass of CO₂ is 44.01 g/mol
The mass of CO₂ released when 13.2 gallons of CH₄ burn is: