Answer: Each chlorine has 7 valence electrons.
Step-by-step explanation: Chlorine is an element with atomic number 17 and thus contains 17 electrons. Valence electrons are the electrons which are present in the valence shell.
Electronic configuration of chlorine:
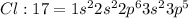
Chlorine atom has 7 valence electrons (2 in 3s and 5 in 3p orbital) and thus need to share one electron with other chlorine atom to complete its octet and attain stability.