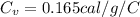At equilibrium, both metal and calorimeter have the same temperature. By conservation of energy, the energy lost by the metal is gained by the calorimeter:
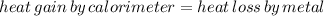
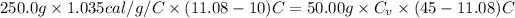
Where
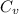
is the specific heat of the unknown metal. Solving this gives us the specific heat of the metal: