Answer: The correct answer is Option D.
Step-by-step explanation:
Number of electrons that are present in an atom is determined by the electronic configuration of that atom.
If an ion is carrying a positive charge, it means that the atom has lost electrons and if an ion is carrying a negative charge, it means that the atom has gained electrons.
For the given options:
Option A: The atomic number of hydrogen atom is 1 and the electronic configuration for
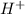 ion will be:
ion will be:
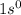
Thus, this atom does not have any electrons.
Option B: The atomic number of bromine atom is 35 and the electronic configuration for
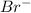 ion will be:
ion will be:
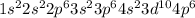
Thus, this atom has 36 electrons.
Option C: The atomic number of aluminium atom is 13 and the electronic configuration for
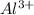 ion will be:
ion will be:
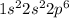
Thus, this atom has 10 electrons.
Option D: The atomic number of calcium atom is 20 and the electronic configuration for
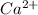 ion will be:
ion will be:
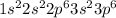
Thus, this atom has 18 electrons.
Hence, the correct answer is option D.