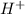Answer : The correct option is,
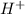
Explanation :
Ions : The ion is formed when the net electric charge is due to the loss or gain of one or more number of electrons.
Element : It is a chemical substance in which has same number of atoms.
Compound : It is defined as the compound is formed from two or more chemical elements in which the elements are chemically bonded to each other. And the elements in the compound are in fixed ratio.
From the given options,
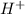 is an ion.
is an ion.
Mg is an element.
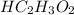 is a compound.
is a compound.
Hence the ion is,