Each row is independent from the other, so we can use the same method for each one.
Le'ts give a variable for each column:
- S: Symbol
- z: Z (Atomic number)
- a: A (Mass number)
- p: No of Protons
- e: No of Electrons
- n: No of Neitrons
- c: Charge
In the symbol column, the right supercript is the charge, so since the Symbol is:
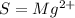
The charge is 2+. We write as 2+, but in math we would write as +2, they are the same, but in different notations.
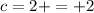
The atomic number and the symbol are always unique, so each element has always the same atomic number and the other way around too.
From a periodic table, we can see which Element corresponds to which atomic number Z.
On a periodic table, we can see that the atomic number of Mg is 12, so:
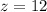
Also, the atomic number and the number of protons is always the same:
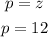
The mass number, a, is alwais the number of protons plus the number of neutrons:
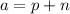
Since we know that the mass number is 25 in this case, we can calculate the number of protons:
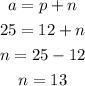
So it checks out with the number on the table.
Each proton has a charge of 1+ and each electron has a charge of 1-. In Math terms, we can say that each proton counts as +1 and each electron counts as -1.
Thus, the charge is the number of protons minus the number o electrons:
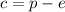
Since the charge is 2+ and there are 12 protons, we can say:
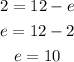
So, there are 10 electrons.
Putting altogether, we have:
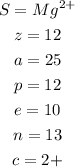
And, by the names:
Symbol: Mg²⁺
Z: 12
A: 25
No. of Protons: 12
No. of Electrons: 10
No. of Neutrons: 13
Charge: 2+