Answer : The pH of the solution is, 3
Solution : Given,
Concentration (C) = 0.06 M
Acid dissociation constant =
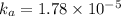
The equilibrium reaction for dissociation of
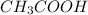 (weak acid) is,
(weak acid) is,
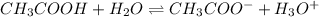
initially conc. c 0 0
At eqm.
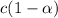
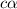
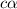
First we have to calculate the concentration of
![[H^+]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/j35v8j284apum01v8otcvhp584v9p1yjwo.png)
As, we know that
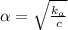 (for weak electrolyte) ...........(1)
(for weak electrolyte) ...........(1)
where,
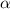 is degree of dissociation
is degree of dissociation
![[H^+]=c\alpha](https://img.qammunity.org/2018/formulas/chemistry/middle-school/z8fscfy7u4tz33b9u18ocr8xjl8289x8i3.png) ..................(2)
..................(2)
By equation both the equations (1) and (2), we get
![[H^+]=√(k_a* c)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/d52dh8g6l25arb1n5j44ns356vik4bkv9z.png)
Now put all the given values in this expression, we get
![[H^+]=\sqrt{1.78* 10^(-5)* 0.06}=1.033* 10^(-3)M](https://img.qammunity.org/2018/formulas/chemistry/middle-school/lmccvapyqi9awobwgvk0kyry3pg0w9hssz.png)
Now we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
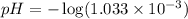
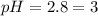
Therefore, the pH of the solution is, 3