Answer:
0.08 mol/L is the concentration of the HCl solution.
Step-by-step explanation:
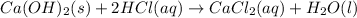
Moles of calcium = 0.00100 mol
According to reaction ,1 mole of calcium hydroxide reacts with 2 moles of HCl.
Then 0.00100 mole of calcium hydroxide will react with:
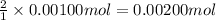
Moles of HCl = 0.00200 mol
Volume of solution = 25.00 mL = 0.025 L
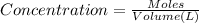
Concentration of HCl solution:
![[HCl]=(0.00200 mol)/(0.025 L)=0.08 mol/L](https://img.qammunity.org/2018/formulas/chemistry/high-school/bkcjg1b5zbmfkmpge1ow3bq0wujjo9k95x.png)