Answer: Option (c) is the correct answer.
Step-by-step explanation:
At equilibrium the relationship expressed between the amounts of concentration of products and reactants in a reversible chemical reaction is known as equilibrium constant.
The concentration of solids and liquids is considered equal to 1 in equilibrium constant.
For example, in the given reaction
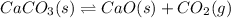
Therefore, its equilibrium constant will be as follows.
![K_(eq) = ([CO_(2)][CaO])/(CaCO_(3))](https://img.qammunity.org/2018/formulas/chemistry/high-school/j14gkrfvehlhs0lwxd9gafc81a98dlqoc7.png)
Here concentration of
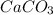 and CaO is 1 as these are solids.
and CaO is 1 as these are solids.
Thus, equilibrium constant expression will be as follows.
![K_(eq) = [CO_(2)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/lwunkv5n1hmiy1s2c2tu1x6j1uko85n1kg.png)