Step-by-step explanation:
It is known that number of moles is equal to the mass divided by molar mass of a substance.
Mathematically, No. of moles =
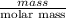
Since it is given that number of moles is 0.80 mol and molar mass of
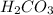 is 62.03 g/mol.
is 62.03 g/mol.
Therefore, we can conclude that mass of
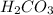 is as follows.
is as follows.
No. of moles =
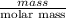
0.80 mol =
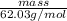
mass = 49.62 g
Thus, we can conclude that mass in grams of 0.800 mole of
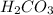 is 49.62 g.
is 49.62 g.