Answer: Option (b) is the correct answer.
Step-by-step explanation:
A solution that contains more number of hydroxide or
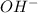 ions is known as a basic solution.
ions is known as a basic solution.
Also, pH of a basic solution is more than 7.
A solution that contains more number of hydrogen or
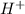 ions is known as an acidic solution.
ions is known as an acidic solution.
Also, pH of a basic solution is less than 7.
A solution that contains equal number of hydroxide and hydrogen ions is known as a neutral solution.
So, pH of a neutral solution is equal to 7.
Thus, we can conclude that basic is the best term which describes a solution that contains more
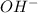 ions than
ions than
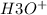 ions.
ions.