Answer: Option (D) is the correct answer.
Step-by-step explanation:
In the equation, number of molecules of carbon, hydrogen and oxygen on the reactant and product side are as follows.
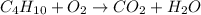
No. of carbon atoms on reactant side = 4
No. of hydrogen atoms on reactant side = 10
No. of oxygen atoms on reactant side = 3
No. of carbon atoms on product side = 1
No. of hydrogen atoms on product side = 2
No. of oxygen atoms on product side = 3
Balance the product side by multiplying no. of carbon atoms with 4 and no. of hydrogen atoms with 5. Therefore, the equation will be as follows.
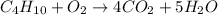
Now, the no. of oxygen atoms on product side is 8 + 5 = 13. The no. of oxygen atoms on reactant side 2.
Thus, multiplying the no. of oxygen atoms on reactant side by
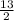 . Therefore, the equation will become as follows.
. Therefore, the equation will become as follows.
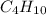 +
+
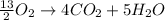
Multiply the whole equation by 2, then the equation will be as follows.
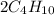 +
+
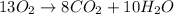
Thus, we can conclude that the coefficient 12 should be changed by Leslie.