Answer:
0.38 moles of hydrogen gas will be produced.
Step-by-step explanation:
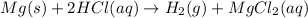
Mole sof HCl = n
Molarity of the HCL solution = 3.0 M
Volume of HCL solution = 250.0 mL = 0.250 L
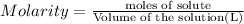
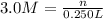
n = 0.75 mol
According to reaction , 2 moles of HCl gives 1 mol of hydrogen gas.
Then 0.75 mol of HCL will give:
 of hydrogen gas.
of hydrogen gas.
0.38 moles of hydrogen gas will be produced from 250.0 milliliters of a 3.0 M HCl in an excess of Mg.