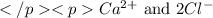Answer: The ions which form calcium chloride compound are
Step-by-step explanation:
Ionic compound is defined as the compound which is formed by complete transfer of electrons from one atom to another atom.
The atom which looses the electron is known as electropositive atom and the atom which gains the electron is known as electronegative atom. This bond is usually formed between a metal (carrying positive charge) and a non-metal (carrying negative charge)
Ionic compound is formed by the attraction of oppositely charged ions.
Calcium chloride is an ionic compound which is formed by the combination of 1 calcium ions
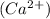 and 2 chloride ions
and 2 chloride ions
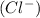
Hence, the ions which form calcium chloride compound are