Answer:
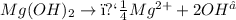
Step-by-step explanation:
Ionization is a type of chemical reaction in which one reactant gives ions on decomposition.
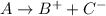
1.
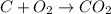 is an example of is an example of synthesis reaction in which two reactants are combining to form one product.
is an example of is an example of synthesis reaction in which two reactants are combining to form one product.
2.
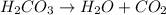 is an example decomposition is a type of chemical reaction in which one reactant gives two or more than two products.
is an example decomposition is a type of chemical reaction in which one reactant gives two or more than two products.
3.
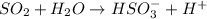 : is an example of synthesis reaction in which two reactants are combining to form one product which being a strong electrolyte dissociate into ions.
: is an example of synthesis reaction in which two reactants are combining to form one product which being a strong electrolyte dissociate into ions.
4.
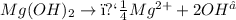 is an example of ionization in which one reactant gives ions on decomposition.
is an example of ionization in which one reactant gives ions on decomposition.