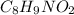The question is incomplete, here is the complete question:
Crime scene investigators keep a wide variety of compounds on hand to help with identifying unknown substances they find in the course of their duties. One such investigator, while reorganizing their shelves, has mixed up several small vials and is unsure about the identity of a certain powder. Elemental analysis of the compound reveals that it is 63.57% carbon, 6.000% hydrogen, 9.267% nitrogen, 21.17% oxygen by mass. Which of the following compounds could the powder be?
a.)
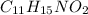 = 3,4-methylenedioxymethamphetamine (MDMA), illicit drug
= 3,4-methylenedioxymethamphetamine (MDMA), illicit drug
b.)
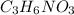 = hexamethylene triperoxide diamine (HMTD), commonly used explosive
= hexamethylene triperoxide diamine (HMTD), commonly used explosive
c.)
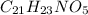 = heroin, illicit drug
= heroin, illicit drug
d.)
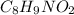 = acetaminophen, analgesic
= acetaminophen, analgesic
e.)
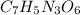 = 2,4,6-trinitrotoluene (TNT), common used explosive
= 2,4,6-trinitrotoluene (TNT), common used explosive
f.)
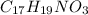 = morphine, analgesic
= morphine, analgesic
g.)
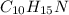 = methamphetamine, stimulant
= methamphetamine, stimulant
h.)
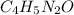 = caffeine, stimulant
= caffeine, stimulant
Answer: The powder could be acetaminophen, analgesic having chemical formula
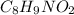
Step-by-step explanation:
We are given:
Percentage of C = 63.57 %
Percentage of H = 6.000 %
Percentage of N = 9.267 %
Percentage of O = 21.17 %
Let the mass of compound be 100 g. So, percentages given are taken as mass.
Mass of C = 63.57 g
Mass of H = 6.000 g
Mass of N = 9.267 g
Mass of O = 21.17 g
To formulate the empirical formula, we need to follow some steps:
- Step 1: Converting the given masses into moles.
Moles of Carbon =
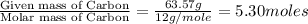
Moles of Hydrogen =
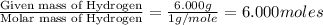
Moles of Nitrogen =
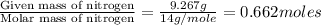
Moles of Oxygen =
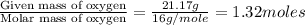
- Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.662 moles.
For Carbon =
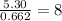
For Hydrogen =
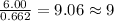
For Nitrogen =
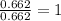
For Oxygen =
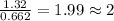
- Step 3: Taking the mole ratio as their subscripts.
The ratio of C : H : N : O = 8 : 9 : 1 : 2
The empirical formula for the given compound is
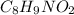
Hence, the powder could be acetaminophen, analgesic having chemical formula