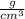Answer:
The density of the iron shot is 7.92
Step-by-step explanation:
Density is a quantity that allows you to measure the amount of mass in a given volume of a substance. So, the expression for the calculation of the density is the quotient between the mass of a body and the volume it occupies:
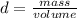
Density is inversely proportional to volume: the smaller the volume occupied by a given mass, the greater the density.
Density, according to the International System of Units, is usually expressed in kilogram per cubic meter
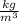 or gram per cubic centimeter
or gram per cubic centimeter
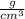 . Although it can also be expressed in any other unit of mass by volume.
. Although it can also be expressed in any other unit of mass by volume.
By adding iron (a solid) to a known volume of water, the level of water in the graduated cylinder increases and the volume of iron is the difference between the final volume and the initial volume of water in ml. This method is called Volume Displacement Measurement of liquids, and is due to the Archimedean principle, which states that "every body immersed in a fluid experiences an upward force called thrust, equivalent to the mass of fluid displaced by the body."
So:
Solid volume = Final volume - Initial volume
In this case:
Volume= 49.1 mL - 45.5 mL= 3.6 mL
Since 1 mL= 1 cm³, 3,6 mL= 3.6 cm³
Then
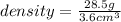
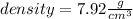
The density of the iron shot is 7.92