Answer: The correct answer is methanol.
Step-by-step explanation:
Molecular compounds are defined as the compounds which are formed by the sharing of electrons between the different atoms that are combining. They do not break easily or the bond between them cannot be broken down easily. Thus, they do not form ions. These molecules are formed when two or more non-metals combine.
Ionic compounds are defined as the compounds which are formed by the donating of electrons from one atom to another. They form ions.
From the given options:
Option 1: Sulfate ion
Chemical formula for this ion is
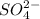 . The given ion is a polyatomic ion and thus is known as ionic compound.
. The given ion is a polyatomic ion and thus is known as ionic compound.
Option 2: Calcium chloride
The chemical formula for this compound is
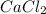 . It is formed by the combination of calcium and chlorine ions. Thus, they will form ionic compound.
. It is formed by the combination of calcium and chlorine ions. Thus, they will form ionic compound.
Option 3: Methanol
The chemical formula for this compound is
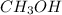 . It is formed by the sharing of electrons between carbon, hydrogen and oxygen atoms. Thus, it is considered as a molecular compound.
. It is formed by the sharing of electrons between carbon, hydrogen and oxygen atoms. Thus, it is considered as a molecular compound.
Option 4: Oxygen
This is formed by the sharing of electrons between two oxygen atoms. Thus, this is considered as a non-polar covalent compound.
Hence, the correct answer is methanol.