Answer:
At 579°C the volume of the gas be three times as big.
Step-by-step explanation:
Under constant pressure:
Initial temperature of the gas =
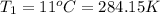
Initial volume of the gas =
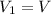
Initial temperature of the gas =
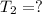
Initial volume of the gas =

Applying Charles law:
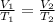 (Constant pressure)
(Constant pressure)
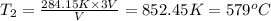
At 579°C the volume of the gas be three times as big.