Answer : The volume of a 1.50 M HCl solution use should be 0.133 L
Explanation :
Using neutralization law,
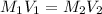
where,
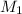 = concentration of HCl solution = 1.50 M
= concentration of HCl solution = 1.50 M
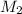 = concentration of another HCl solution = 0.100 M
= concentration of another HCl solution = 0.100 M
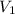 = volume of HCl solution = ?
= volume of HCl solution = ?
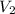 = volume of another HCl solution = 2.00 L
= volume of another HCl solution = 2.00 L
Now put all the given values in the above law, we get:
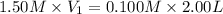
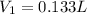
Therefore, the volume of a 1.50 M HCl solution use should be 0.133 L