Answer: Option (c) is the correct answer.
Step-by-step explanation:
When acidity or alkanity of water soluble substances is measured then it is known as pH.
Mathematically, pH =
![-log [H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/yhuny7f00oxtjxsdmnkts3zwu0fowf3edy.png)
where
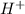 = concentration of hydrogen ions.
= concentration of hydrogen ions.
Therefore, we can conclude that out of the given options, relationship between the pH number and the power of 10 used to express the [H+] is that pH = the negative of the exponent of concentration.