Answer:
The hydrocarbon has a double C-C bond.
Step-by-step explanation:
Degree of unsaturation is a calculation that allows to know the number of rings and π-bonds.
For a hydrocarbon of formula CₓHₙ, the degree of unsaturation is:
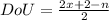
For C₆H₁₂:
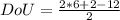 = 1.
= 1.
As the hydrocarbon is a straight-chain hydrocarbon, there are not rings, thus, degree of unsaturation is due 1 π-bond, that means that hydrocarbon has a double C-C bond.
I hope it helps!