Answer: Each isotope of an element has different mass number.
Step-by-step explanation:
Isotope is defined as the chemical specie which belong to the same element but differ in their molecular mass.
Atomic number is defined as the number of protons or electrons that are present in a neutral atom.
Atomic number = number of protons = number of electrons
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
This also means that the chemical species have same number of protons and electrons but different number of neutrons.
For Example: Isotopes of chlorine, Cl-35 and Cl-37
For
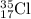 isotope:
isotope:
Number of protons = 17
Number of neutrons = 35 - 17 = 18
For
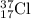 isotope:
isotope:
Number of protons = 17
Number of neutrons = 37 - 17 = 20
Hence, each isotope of an element has different mass number.