Step-by-step explanation:
Helium is the element of 18th group and first period. The electronic configuration of helium is - 2 or
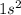
There are 2 valence electrons of helium.
Fluorine is the element of 17th group and second period. The electronic configuration of fluorine is - 2, 7 or
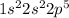
There are 7 valence electrons of fluorine.
Sodium is the element of 1st group and third period. The electronic configuration of sodium is - 2, 8,1 or
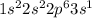
There is 1 valence electron of sodium.
Potassium is the element of 1st group and forth period. The electronic configuration of potassium is - 2, 8, 8, 1 or
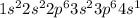
There is 1 valence electron of potassium.
Hence,
He: 2,0,0,0,0,0,0
F: 2,7,0,0,0,0,0
Na: 2,8.1,0,0,0,0
K: 2,8,8,1,0,0,0