Answer: Option (B) is the correct answer.
Step-by-step explanation:
The
![[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tixcvmiul05vtrn8s7qstptt69zx1u6brh.png) and
and
![[OH^(-)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/5jolflf7yijryo1qblzyptwrqcnvz4t32k.png) are interdependent on each other that is if the concentration of
are interdependent on each other that is if the concentration of
 ions is high then the concentration of
ions is high then the concentration of
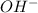 ions is low and vice versa.
ions is low and vice versa.
The pH of a substance can be calculated as follows.
pH = -log
![[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tixcvmiul05vtrn8s7qstptt69zx1u6brh.png)
This means when the concentration of
 ions is high then the pH of the solution or substance will be low.
ions is high then the pH of the solution or substance will be low.
It is also known that pH + pOH = 14, or pOH = 14 - pH.
Thus, we can conclude that when the pH is low then the pOH will be high and vice versa.
Therefore, option (B) is the correct answer that is a low [OH– ] and a high pOH.