Answer: Option (C) is the correct answer.
Step-by-step explanation:
A compound formed by transfer of electrons between its atoms is known as an ionic compound.
Ionic compound when dissolve in water or polar solvent then they dissociate into ions and thus they are able to conduct electricity. Therefore, this type of solution is known as an electrolyte.
Whereas a covalent compound does not dissociate into ions. Hence, it is not able to conduct electricity. Thus, it does not act as an electrolyte.
Therefore, we can conclude that
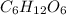 form a non-electrolyte solution in water.
form a non-electrolyte solution in water.