Answer:
The heat released = 12.2 kJ
Step-by-step explanation:
The heat of fusion of water = 6.01kJ/mol
Molar mass of water = 18g
Thus we can say that
The energy released when 18g (molar mass of water) freeze = 6.01kJ
thus the heat released when one gram of water freeze
=
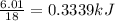
The heat released when 36.5g of water freeze = 0.3339 X 36.5 = 12.187 kJ