Answer : The element that has no same number of electrons as of argon will be, Neon (Ne).
Explanation :
The element is, Argon
The atomic number of argon = 18
The electronic configuration of argon is,
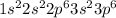
The number of electrons in argon = 18
The element is,
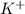
The atomic number of K (potassium) = 19
The electronic configuration of 'K' is,
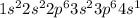
The electronic configuration of
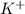 is,
is,
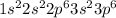
The number of electrons in
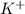 = 18
= 18
The element is,
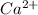
The atomic number of Ca (calcium) = 20
The electronic configuration of 'Ca' is,
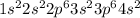
The electronic configuration of
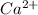 is,
is,
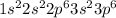
The number of electrons in
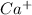 = 18
= 18
The element is, Neon
The atomic number of neon= 10
The electronic configuration of neon is,
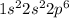
The number of electrons in neon = 10
The element is,
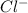
The atomic number of Cl (chlorine) = 17
The electronic configuration of 'Cl' is,
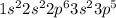
The electronic configuration of
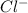 is,
is,
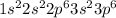
The number of electrons in
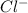 = 18
= 18
The element is,
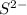
The atomic number of S (sulfur) = 16
The electronic configuration of 'S' is,
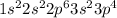
The electronic configuration of
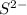 is,
is,
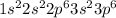
The number of electrons in
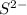 = 18
= 18
Therefore, the element that has no same number of electrons as of argon will be, Neon (Ne).