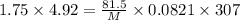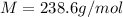Answer: Molar mass of gas is 238.6 g/mol
Step-by-step explanation:
Using ideal gas equation:
PV = nRT
P= pressure = 1.75 atm
V= volume = 4.92 L
n = no of moles =
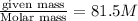
R= gas constant =0.0821 Latm\molK
T = temperature = 307 K
R= gas constant = 8.314 J/Kmol
T= temperature = 190.7 K
M= molecular mass of gas = ?g/mol