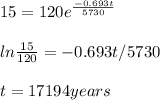Answer:
Time taken for the C-14 to decay from 120 to 15 g = 17194 years
Step-by-step explanation:
Given:
Half life of C-14, t1/2 = 5730 years
Initial amount of C-14, N(0) = 120 g
Amount of C-4 after time t , N(t) = 15 g
Formula:
Radioactive decay is given by the following expression
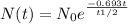
Substituting the values of N(t), N(0), t1/2 we get: