Answer:
Concentration of
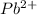 decreases
decreases
Step-by-step explanation:
In a saturated solution of
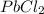 , the following solubility equilibrium exist-
, the following solubility equilibrium exist-
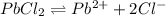
Now, NaCl contains common ion
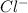 . Therefore, concentration of
. Therefore, concentration of
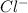 increases in solution.
increases in solution.
But, at a particular temperature, equilibrium constant remain constant. Therefore to keep equilibrium constant same
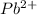 will reacts with excess
will reacts with excess
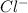 to decrease concentration of
to decrease concentration of
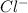 .
.
Therefore concentration of
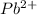 decreases.
decreases.
Note: Equilibrium constant for the above equilibrium=
![\left [ Pb^(2+) \right ]\left [ Cl^(-) \right ]^(2)](https://img.qammunity.org/2018/formulas/chemistry/high-school/pqzq0dsh058avp94i4ip90aqte8z6oelo9.png)