Answer: The standard reduction potential of hydrogen is taken as 0.00V.
Step-by-step explanation:
The standard reduction potential is defined as the tendency of a chemical species to get reduced. It is denoted as
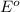 . This value is measured in volts.
. This value is measured in volts.
More the positive value of the potential of an element, it is more likely to get reduced.
Standard reduction potential is the relative potential to standard hydrogen electrode which is taken as 0.00V.
Hence, the standard reduction potential of hydrogen is taken as 0.00V.