Answer : The total pressure in the flask is, 34.9 torr
Explanation :
As we know that the total pressure is the sum of the partial pressure of the gases.
The formula used is :
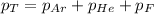
where,
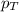 = total pressure in the flask = ?
= total pressure in the flask = ?
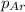 = partial pressure of argon = 14.1 torr
= partial pressure of argon = 14.1 torr
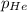 = partial pressure of helium = 12.2 torr
= partial pressure of helium = 12.2 torr
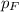 = partial pressure of fluorine = 8.6 torr
= partial pressure of fluorine = 8.6 torr
Now put all the given values in the above formula, we get
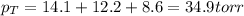
Therefore, the total pressure in the flask is, 34.9 torr