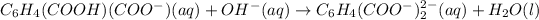Step-by-step explanation:
The formula unit pf potassium phthalate is
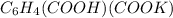
The reaction between potassium hydrogen phthalate and cesium hydroxide.
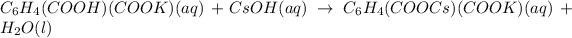
In the above reaction, proton will get combine with hydroxide ion from cesium hydroxide to form a water molecule.
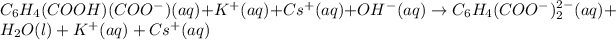
Removing common ions from both sides :
The net ionic equation is :