The chemical reaction equation for this is
XeF6 + 3H2 ---> Xe + 6HF
Assuming gas behaves ideally, we use the ideal gas formula to solve for number of moles H2 with T = 318.15K (45C), P = 6.46 atm, V = 0.579L. Then we use the gas constant R = 0.08206 L atm K-1 mol-1.
we get n = 0.1433 moles H2
to get the mass of XeF6,
we divide 0.1433 moles H2 by 3 since 1 mole XeF6 needs 3 moles H2 to react then multiply by the molecular weight of XeF6 which is 245.28 g/mole XeF6.
0.1433 moles H2 x
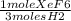
x
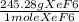
= 11.71 g XeF6
Therefore, 11.71 g of XeF6 is needed to completely react with 0.579 L of Hydrogen gas at 45 degrees Celcius and 6.46 atm.