Answer:
Over 140 times as great the mass of the sugar in regular soda as the mass of the sweetener in diet soda.
Explanation:
In Regular Soda
Volume of sugar =
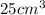
Density of table sugar =
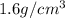
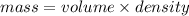
So, Mass of Sugar in regular soda =
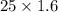
=
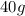
In Diet Soda
Volume of aspartame =
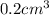
Density of aspartame =
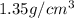
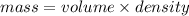
So, Mass of aspartame in diet soda =
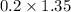
=
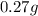
So, now to find How many times as great is the mass of the sugar in regular soda as the mass of the sweetener in diet soda:
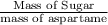
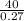
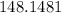
Hence Over 140 times as great the mass of the sugar in regular soda as the mass of the sweetener in diet soda.
Thus Option D is correct.