Answer : The concentration of sodium ions in a
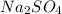 solution is 0.20 M
solution is 0.20 M
Explanation :
The dissociation reaction of
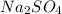 in solution will be:
in solution will be:
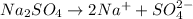
From the reaction we conclude that, 1 mole of
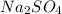 dissociate in solution to give 2 moles of
dissociate in solution to give 2 moles of
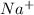 ion (sodium ion) and 1 mole of
ion (sodium ion) and 1 mole of
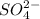 ion (sulfate ion).
ion (sulfate ion).
As per question,
0.10 M of
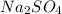 dissociate in solution to give
dissociate in solution to give
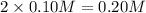 of
of
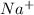 ion (sodium ion) and 0.10 M of
ion (sodium ion) and 0.10 M of
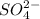 ion (sulfate ion).
ion (sulfate ion).
Hence, the concentration of sodium ions in a
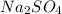 solution is 0.20 M
solution is 0.20 M