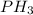Answer: The chemical formula of phosphine is
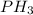
Step-by-step explanation:
Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound. These are usually formed when two non-metals react.
Phosphine is the chemical compound which is formed by the combination of phosphorus and hydrogen atoms.
Electronic configuration of phosphorus is
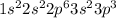
Phosphorus has empty d-orbital in its shell. So, it can form 5 bonds. As, hydrogen has small size, it will form only 3 bonds with phosphorus.
Hence, the chemical formula for phosphine will be