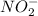Answer: The reducing agent in the given reaction is
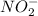
Step-by-step explanation:
Reducing agents are defined as the agents which reduces the other substance and itself gets oxidized. These agents undergoes oxidation reactions and oxidation reaction is defined as the reaction in which an atom looses its electrons.
Oxidizing agents are defined as the agents which oxidize other substance and itself gets reduced. These agents undergoes reduction reactions and reduction reaction is the reaction in which an atom gains electrons.
For the given chemical reaction:
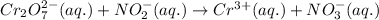
The half reactions for the above equation follows:
Oxidation half reaction:
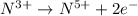 ( × 3)
( × 3)
Reduction half reaction:
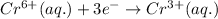 ( × 2)
( × 2)
In the above reaction, the reactant which is getting oxidized is
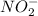 . So, it is the reducing agent.
. So, it is the reducing agent.
Hence, the reducing agent in the given reaction is