Answer:
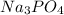
Step-by-step explanation:
Depression in freezing point is given by:
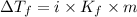
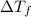 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor
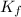 = freezing point constant
= freezing point constant
m= molality =
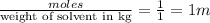
1. For
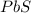
, i= 1 as it is a non electrolyte and does not dissociate.
2. For
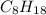
, i= 1 as it is a non electrolyte and does not dissociate.
3. For
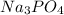
, i= 4 as it is a electrolyte and dissociate to give 4 ions.

4. For
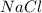
, i= 2 as it is a electrolyte and dissociate to give 2 ions.

Thus as vant hoff factor is highest for
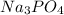 and the freezing point will be most affected.
and the freezing point will be most affected.