Step-by-step explanation:
Second energy level consists of one "s" sub-shell and a "p" sub-shell. So, in a "s" sub-shell there is only one orbital. Whereas in a "p" sub-shell there are 3 orbitals which are
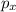 ,
,
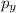 , and
, and
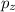 .
.
Also, each orbital can contain a maximum of two electrons.
Therefore, we can conclude that in total there are four orbitals (one from s and three from p) found in the second energy level.