Answer:
Gold(II) sulphate is soluble
Step-by-step explanation:
According to solubility rule, all sulphates are soluble except
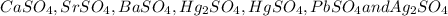
One of the reason for gold sulphate to be a soluble salt is that both gold(II) and sulphate are bigger in size. Therefore their lattice energy is low as lattice energy is inversely proportional to sum of radius of cation and anion.
Hence lattice structure of gold sulphate can be easily disrupted and make constituting ions to get soluble.